how many valence electrons are in zirconium|Zirconium : Cebu Mar 23, 2023 Play chess online for free on Chess.com with over 150 million members from around the world. Have fun playing with friends or challenging the computer!
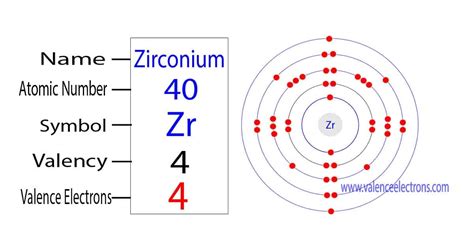
how many valence electrons are in zirconium,The elements that have 1, 2, or 3 electrons in the last shell donate the electrons in the last shell during bond formation. The elements that form bonds by donating electrons are called cations. Zirconium donates the electron of the last shell to form bonds and turns into a zirconium ion(Zr4+). That is, . Tingnan ang higit paThe 2nd element in group-4 is zirconium. The elements in groups 3-12 are called transition elements. The valence electrons are the total number of electrons in the last orbit. But . Tingnan ang higit paThe valence electrons have to be determined by following a few steps. The electron configuration is one of them. It is not possible to determine the valence electron . Tingnan ang higit pahow many valence electrons are in zirconiumThe ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). The number of . Tingnan ang higit pa
Mar 23, 2023

This table of element valences includes the maximum valence and most .Zirconium This table of element valences includes the maximum valence and most .
how many valence electrons are in zirconium Zirconium This table of element valences includes the maximum valence and most . Zirconium has four valance electrons, with two in the 4d level and two in the 5s level. This allows it to combine with other elements and ions in different . The valency of zirconium is useful in figuring out the combining capacity of elements. You can also refer to the periodic table to cross-check Zirconium’s valency. . The electron configuration indicates that the zirconium-ion has adopted the electron configuration from krypton. The valence electrons (Zr 4+) of the zirconium-ion have eight electrons since the .
Zirconium is a chemical element of the periodic table with chemical symbol Zr and atomic number 40 with an atomic weight of 91.2242 u and is classed as transition metal and is . Wayne Breslyn. 754K subscribers. 141. 28K views 3 years ago. A step-by-step description of how to write the electron configuration for Zirconium (Zr). In order to .1789. Discovered by. Martin Heinrich Klaproth. Origin of the name. The name is derived from the Persian, 'zargun', meaning gold coloured. Allotropes. - Zr. Zirconium. 40. .Zirconium. 40. 91.224. Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. PeriodA horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right.
The electron numbers and bond energies on the strongest covalent bonds in the m-ZrO 2 phase were the greatest, the values were 0.901106 and 157.5933 kJ/mol, respectively. Those in the t-ZrO 2 phase took second place, which were 0.722182 and 123.9304 kJ/mol, and those in the c-ZrO 2 phase were the smallest, which were . The valency of Zr is 4 valence electrons in its outer shell. The numbers of valence electrons in the outer shell of zirconium are exactly its valency. The valency of zirconium is useful in figuring out the combining capacity of elements. You can also refer to the periodic table to cross-check Zirconium’s valency.The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z. Electron configuration of Zirconium is [Kr] 4d2 5s2. Possible oxidation states are +4.
Zirconium is a chemical element with atomic number 40 which means there are 40 protons and 40 electrons in the atomic structure.The chemical symbol for Zirconium is Zr. Electron Configuration and Oxidation States of Zirconium. Electron configuration of Zirconium is [Kr] 4d2 5s2. Possible oxidation states are +4. Electron . This table of element valences includes the maximum valence and most common valence values. Use this is a reference with a periodic table. . Here is a table of element valences. Remember that an element's electron cloud will become more stable by filling, emptying, or half-filling the shell. . Zirconium (+2), (+3), +4: 41: Niobium (+2), +3 .Zirconium is a chemical element of the periodic table with chemical symbol Zr and atomic number 40 with an atomic weight of 91.2242 u and is classed as a transition metal. . Valence electrons : 4: Valency electrons : 4: Bohr model: Electron shell for Zirconium, created by Injosoft AB Zr. Figure: Shell diagram of Zirconium (Zr) atom. Orbital . Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. . Zirconium (Zr) 4: 41: Niobium (Nb) 5: 42: Molybdenum (Mo) 6: 43: Technetium (Tc) 7: 44: Ruthenium (Ru) 8: 45: Rhodium (Rh) 9: 46: Palladium (Pd) 10: 47: Silver (Ag) 11: 48: .How many valence electrons does Zirconium have? How many unpaired electrons are in an iron atom in the ground state? a) 0 b) 3 c) 4 d) 5 e) 6 Explain your answer. How many unpaired electrons are in the electron structure of _{24}Cr? What is the number of valence electrons, core electrons, and unpaired electrons in the ground state of carbon? sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group . Here in this problem we have to find out how many uncleared electrons are there in zirconia for bless now, the first we have to write the electron configuration for the zirconia, Zirconium has atomic number 40. . An atom has a valence electron configuration of 7s25f13. How many unpaired electrons are there in this atom?
How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the answer is three. In fact, the number of valence electrons goes up by one for each step across a period, until the last element is reached. Neon, with its configuration ending in \(2s^2 2p .
Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .
The electron configuration of rubidium shows that there is an unpaired electron(5s 1) in the last orbit of rubidium.Therefore, the valency of rubidium is 1. How many valence electrons does rubidium ion(Rb +) have?. The elements that have 1, 2, or 3 electrons in the last shell donate the electrons in the last shell during bond formation.
How many electrons does zirconium? Zirconium is a metal element. There are 40 electrons in a single atom. . How many valence electrons are in zirconium atom? protons:40electron:40neutrons: can .Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..

Since the atomic number of zirconium is 40, the total electrons of zirconium are 40. Second, make a table of subshell and its maximum electrons. Calculate the maximum number of electrons each subshell can hold using the formula: 4ℓ + 2. Where, ℓ = azimuthal quantum number of the subshell. For s subshell, ℓ = 0. When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. For example, fluorine has seven valence electrons, so it is most .
how many valence electrons are in zirconium|Zirconium
PH0 · Zirconium Valence Electrons
PH1 · Zirconium (Zr)
PH2 · Zirconium
PH3 · Valences of the Elements Chemistry Table
PH4 · Valence Electrons Chart for All Elements
PH5 · How to Write the Electron Configuration for Zirconium (Zr)
PH6 · How to Find the Valence Electrons for Zirconium (Zr)?
PH7 · How many valence electrons does zirconium (Zr) have?
PH8 · How many valence electrons does Zirconium (Zr) have?
PH9 · How Many Valence Electrons Does Zirconium Have?
PH10 · Complete Electron Configuration for Zirconium (Zr, Zr4+)